What Causes Diamond Color?
Only about 1 in 10,000 natural gem quality diamonds is fancy colored. Within that small percentage, some colors are more common than others, making certain of the colors extraordinarily rare. So, what causes us to see these various colors, and why are some so rare?
Let’s start with how we perceive color as human beings.
Visible light includes a series of component colors between infrared light, which is above 700 nanometers, and ultraviolet light, which is below 380 nanometers. What your naked eye perceives as white light is actually all visible colors combined, or all the wavelengths of the visible spectrum coming together. When all of those wavelengths enter the eye at once, we see them together as white light.

- Fun Fact #1: Sir Isaac Newton originally identified seven hues in the visible spectrum: Red, orange, yellow, green, blue, indigo, and violet. Those seven were traditionally taught as the acronym “ROY G BIV.” However, when viewing a rainbow, very few people distinguish a hue between blue and violet, so modern references to the spectrum often shorten the list to red, orange, yellow, green, blue and violet.
White or Black = Full Reflection or Full Absorption
- FF #2: Any white object we see is simply reflecting back all hues in the visible spectrum. So, white is all colors reflected.
- FF #3: Likewise, any black object we see is absorbing or “eating” all hues in the visible spectrum. So, black is all colors absorbed. This is why you might burn your skin, sitting on a black leather seat in a black car on a hot day. It absorbs and stores the heat of all wavelengths of visible light coming from the sun.

Color = Selective Absorption
Any colored object we see is simply absorbing or “eating” certain colored wavelengths, selectively, from white light. That results in the object transmitting or reflecting the other colored rays more or less strongly.
- FF #4: The best example of selective color absorption may be plant life. Plant life absorbs every color wavelength except… You guessed it. Green. All other wavelengths remain within the plant. The only wavelengths being transmitted to your eye from the plant are green.
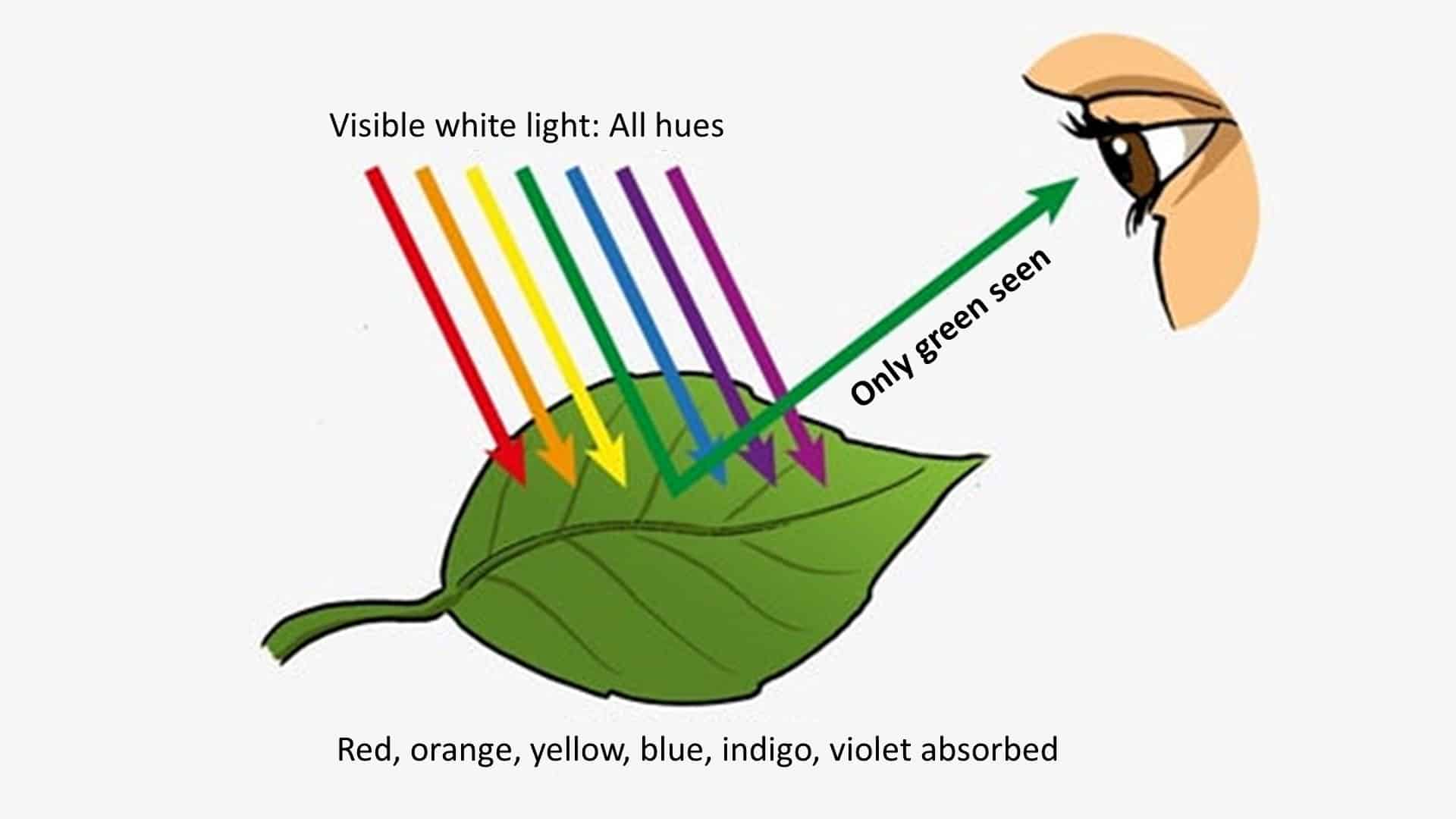
Color in Diamonds
- FF #5: When white light enters a diamond it starts breaking down into its component hues, like a prism. If none of those colors are absorbed and they all wind up exiting the diamond, we see the diamond as white, or colorless.
- FF #6: Whenever we see color in a diamond, it’s due to a combination of the visible hues which are being selectively absorbed by the diamond material, versus those which successfully transmit through the diamond to reach your eye.
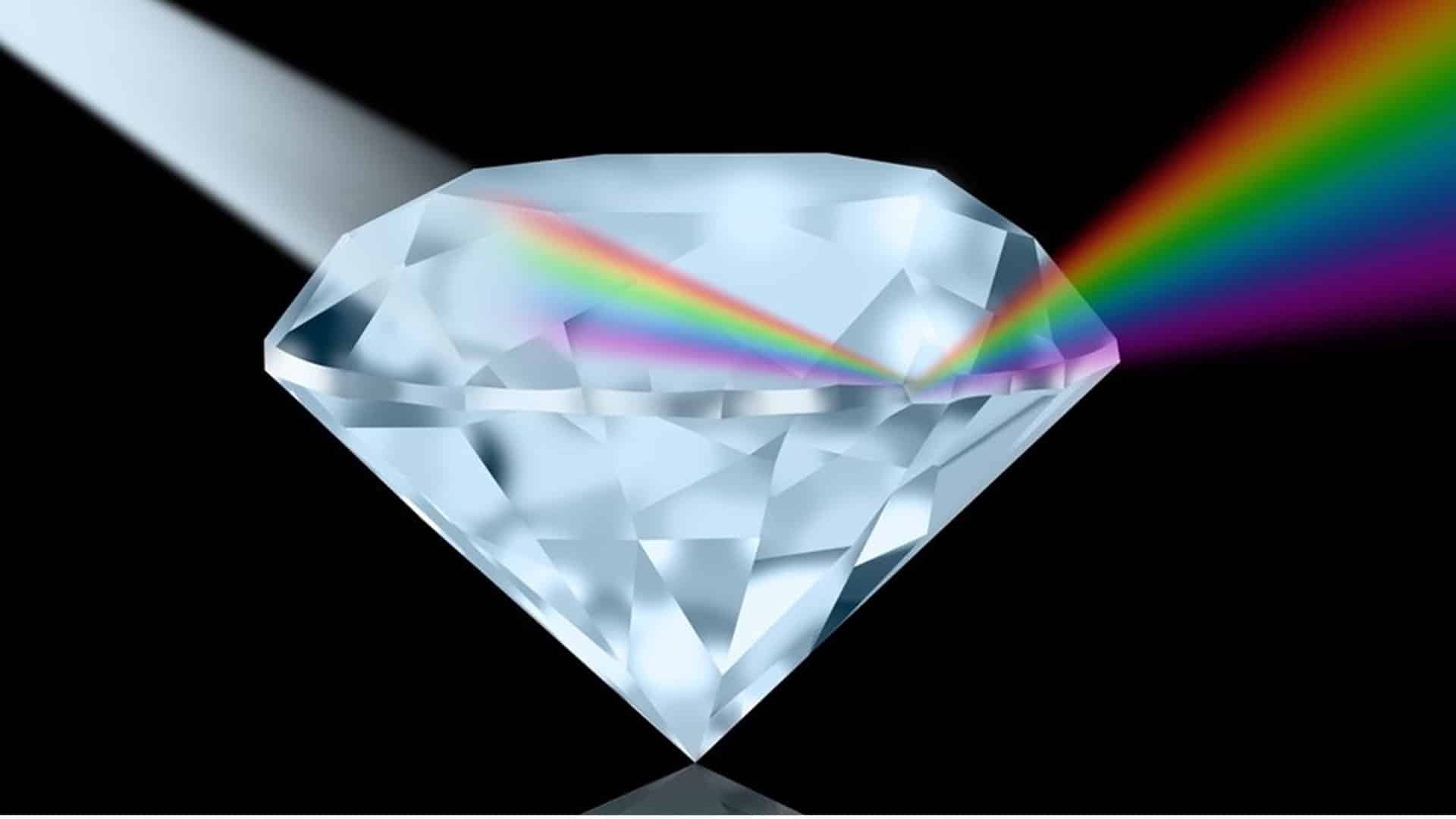
Scientifically speaking, this is how IGI gemologists determine why a diamond shows a certain color. And it’s really interesting to know why a diamond looks yellow, pink, green, etc. Let’s continue with more fun facts.
Yellow, Orange and Brown
Yellow, orange and brown are caused by nitrogen, the most common of non-carbon atoms found in diamonds.
- FF #7: When nitrogen absorption occurs at low wavelengths the color is yellow.
- FF #8: When it occurs at higher wavelengths the color becomes orange (with some exceptions).
- FF #9: Brown is also caused by nitrogen along with another absorption causing feature, which are defects in the crystal lattice.

Gray
- FF #10: We see gray due to the presence of hydrogen, or sometimes boron. And speaking of boron, one of the rarest and most valuable natural diamond colors is also caused by boron. That would be…

Blue
- FF #11: Fancy blue diamonds. They are very rare in nature, but they exist. Boron in a diamond primarily absorbs yellow light. When that yellow is canceled out, it permits us to see the blue wavelengths.

Purple
It may surprise you to know there is not a decisive explanation for what causes us to see purple in natural diamonds. Hydrogen or boron can be present, just as with blue or gray, but we suspect that extremes of pressure also compressed the crystal lattice in a way that causes the blue or gray transmission to change to purple. Bringing us to…
- FF #12: Whenever you see a purple diamond you can tell your friends that the reason behind its color remains one of the very few unsolved mysteries of the gemological world.

Pink and Red
Pink and red in diamonds are not caused by chemical impurities.
- FF #13: As we suspect influences purple, pink and red are caused by deformation of the crystal lattice. Incredible pressures during the growth cycle fostered structural defects that cause pink to red light to be transmitted.
- FF #14: The greater the pressure, the greater the deformation, the greater the color saturation. This is why red diamonds are only found at small carat weights. The pressure which gave them such high saturation also prevented them from growing large.

Green
Green also has a unique color foundation.
- FF #15: Green natural diamond color can be caused by exposure to atomic radiation in the earth where the diamond formed. With that said, it’s possible for nitrogen, hydrogen or nickel atoms to cause green transmission as well.
- FF #16: Most greens have hints of yellow, blue, or gray, making pure green diamonds extremely valuable. In 2016, the 5.03 carat fancy vivid Aurora Green, at bottom right, was sold by Christie’s for more than $16 million.

Black
Black is the absorption of all light.
- FF #17: In natural diamonds, black color can be caused by the heavy presence of inclusions. Other natural black diamonds are heat treated to improve their appearance. And finally, there is another kind of black diamond which is something rather special…

Carbonado
This diamond material has been found in alluvial deposits in Brazil and Central Africa, in areas where there are no diamond bearing rocks. In December 2006 Astrophysicists discovered that carbonado has existed long before the Earth was born. Meaning…
- FF #18: The earth’s supply of carbonado was a special diamond delivery, crashing onto our planet from somewhere in interstellar space. Literally, from out of this world. Read more: “Meteorites and diamonds from space.“

Space Diamonds
- FF #19: The most famous carbonado may be this 555.55 carat giant named “The Enigma.” It was sold in 2022 by Sotheby’s London for $4.3 million dollars. Read more about The Enigma.

Fancy Colored Diamond Grading
- FF #20: Unlike D-Z color grading, where the diamond is viewed upside down, through the side, fancy colored diamonds are graded against a white background, viewed from the top, assessing the stone’s appearance in three critical areas: Hue, tone, and saturation.

Hue, tone and saturation
- FF #21: Hue describes the stone’s overall color. Tone and saturation work together. Tone is the lightness or darkness of the color present. As it gets lighter it becomes hard to detect. As it gets deeper or darker the hue is difficult to read. Saturation refers to the strength, purity, or intensity of the color present. Here are increasing saturations of pink from left to right. With light tone at the top and dark tone at the bottom.

The Three-Dimensional Diamond Color Universe
Considered together, we use a diamond’s hue, tone and saturation to map its precise location in this three-dimensional color universe.

If you’re interested in more fun facts, intelligence and insight from IGI experts and prominent guest authors, be sure to subscribe to the IGI GemBlog, at top right.

Leave a Reply
Want to join the discussion?Feel free to contribute!